Aluminum Chloride Theoretical Yield . In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web this chemistry video tutorial explains how to calculate the percent. Web what is theoretical yield? This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web this theoretical yield calculator will answer all the burning questions you have regarding how to.
from www.slideserve.com
Web this chemistry video tutorial explains how to calculate the percent. Web this theoretical yield calculator will answer all the burning questions you have regarding how to. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web what is theoretical yield? Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s.
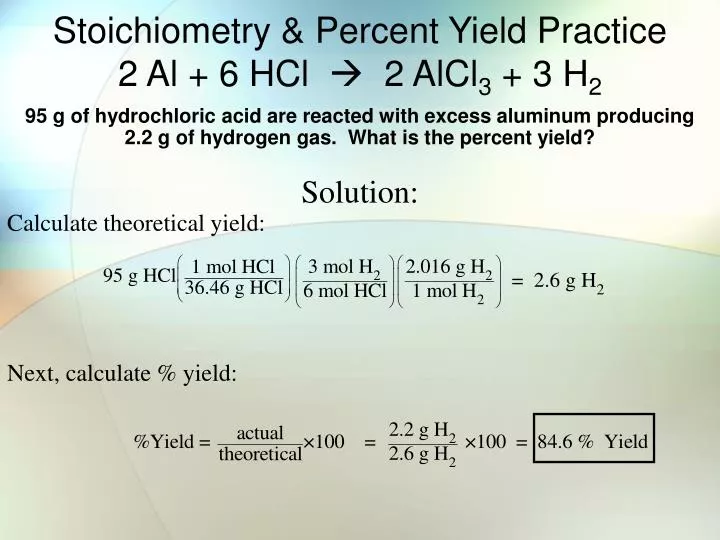
PPT Stoichiometry & Percent Yield Practice 2 Al + 6 HCl 2 AlCl 3 + 3
Aluminum Chloride Theoretical Yield Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web this theoretical yield calculator will answer all the burning questions you have regarding how to. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web this chemistry video tutorial explains how to calculate the percent. In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web what is theoretical yield?
From www.youtube.com
Aluminium reacts with chlorine gas to form aluminium chloride via the Aluminum Chloride Theoretical Yield Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web this. Aluminum Chloride Theoretical Yield.
From mackenzie-chapter.blogspot.com
Aluminum Copper Ii Chloride Reaction Lab Answers 50+ Pages Summary Doc Aluminum Chloride Theoretical Yield Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web each reactant amount is. Aluminum Chloride Theoretical Yield.
From www.chegg.com
Solved Copper (II) Chloride and Aluminum Reaction (done Aluminum Chloride Theoretical Yield Web this chemistry video tutorial explains how to calculate the percent. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web what. Aluminum Chloride Theoretical Yield.
From oneclass.com
OneClass What is the theoretical yield of aluminum oxide if 3.60 mol Aluminum Chloride Theoretical Yield Web this theoretical yield calculator will answer all the burning questions you have regarding how to. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web what is theoretical yield? Web in this lab, students react. Aluminum Chloride Theoretical Yield.
From brainly.in
aluminium burns in chlorine to form aluminium chloride.it's urgent Aluminum Chloride Theoretical Yield Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web what is theoretical yield? In the dynamic landscape of chemical reactions, theoretical yield stands. Aluminum Chloride Theoretical Yield.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Aluminum Chloride Theoretical Yield Web this chemistry video tutorial explains how to calculate the percent. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web what is theoretical yield? Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. This represents a 3:2 (or 1.5:1) ratio of. Aluminum Chloride Theoretical Yield.
From www.numerade.com
SOLVED What is the limiting reagent when 3.5 g of aluminum hydroxide Aluminum Chloride Theoretical Yield Web this chemistry video tutorial explains how to calculate the percent. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web what is theoretical yield? Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web the actual yield is the amount of product that is actually formed. Aluminum Chloride Theoretical Yield.
From www.numerade.com
SOLVED a. Aluminum metal reacts with copper (II) chloride solution to Aluminum Chloride Theoretical Yield Web this theoretical yield calculator will answer all the burning questions you have regarding how to. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web each reactant amount is used to separately calculate the amount. Aluminum Chloride Theoretical Yield.
From www.nagwa.com
Question Video Calculating the Percentage Yield for the Reaction of Aluminum Chloride Theoretical Yield Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. This represents a. Aluminum Chloride Theoretical Yield.
From quizdemurrages.z21.web.core.windows.net
How To Determine Theoretical Yield Aluminum Chloride Theoretical Yield Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web this chemistry video tutorial explains how to calculate the percent. Web the actual yield is the amount of product that is actually formed. Aluminum Chloride Theoretical Yield.
From printablelibbrained.z13.web.core.windows.net
Calculate Percent Yield And Theoretical Yield Aluminum Chloride Theoretical Yield Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. In the dynamic landscape of chemical reactions, theoretical. Aluminum Chloride Theoretical Yield.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to produce aluminum chloride Aluminum Chloride Theoretical Yield Web this chemistry video tutorial explains how to calculate the percent. Web what is theoretical yield? Web this theoretical yield calculator will answer all the burning questions you have regarding how to. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web each reactant amount is used to separately calculate the amount of product that would be formed per. Aluminum Chloride Theoretical Yield.
From www.chegg.com
Solved Synthesis of tpentyl chloride Theoretical yield Aluminum Chloride Theoretical Yield This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web this theoretical yield calculator will answer all the burning questions you have regarding how to. Web the actual yield is the amount of product that is actually formed when the reaction is carried out. Aluminum Chloride Theoretical Yield.
From byjus.com
61. During electrolysis of fused aluminium chloride 0.9 g of aluminium Aluminum Chloride Theoretical Yield Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting reactant. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web this theoretical yield calculator will answer all the burning questions you have regarding how to. In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon. Aluminum Chloride Theoretical Yield.
From www.numerade.com
SOLVED Write the chemical formula for aluminum chloride Answer Use Aluminum Chloride Theoretical Yield Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web what is theoretical yield? Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web the actual yield is the amount of product that is actually formed when the reaction is carried out. Aluminum Chloride Theoretical Yield.
From www.numerade.com
SOLVED 7.Chloroform,CHCl3, reacts with chlorineCl2,to form carbon Aluminum Chloride Theoretical Yield In the dynamic landscape of chemical reactions, theoretical yield stands as a beacon of what could be. Web the actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web in this lab, students. Aluminum Chloride Theoretical Yield.
From www.coursehero.com
[Solved] During an experiment, the percent yield of calcium chloride Aluminum Chloride Theoretical Yield This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web this chemistry video tutorial explains how to calculate the percent. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web the actual. Aluminum Chloride Theoretical Yield.
From www.coursehero.com
[Solved] Calculate the theoretical yield of water produced, in grams Aluminum Chloride Theoretical Yield Web each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s. Web for example, imagine combining 3 moles of h 2 and 2 moles of cl 2. This represents a 3:2 (or 1.5:1) ratio of hydrogen to. Web in this lab, students react copper(ii) chloride with aluminum to determine the limiting. Aluminum Chloride Theoretical Yield.